NCERT solutions: Class 6: Science: Separation of substances
Table of Contents
ToggleIntroduction: Separation of substances
In our day-to-day life, we see many instances of separation of substances from a mixture of materials like:
Separating tea leaves from the tea with the help of strainer

Separating butter from the milk or curd

Separating grains from stalks during harvesting

Do you know?
What is Separation?
A separation is a process that converts a mixture or solution of chemical substances into two or more distinct product mixtures.
Why do we need to separate the substances?
The main purpose of separating the substances are:
- To separate two different, but useful components like separating stones from rice
- To remove non-useful components like churning milk to obtain butter
- To remove impurities or harmful components like separating tea leaves from the tea.
Before moving further, let`s know more about a mixture?
- A mixture contains particles of two or more substances which may be present in the mixture in any ratio. Hence, their composition is variable.
- When we mix two substances, then the properties of the substances do not change in the mixture. They remain intact.
- Air, sugar in water, pebbles in sand, dust in grains are all examples of mixtures.
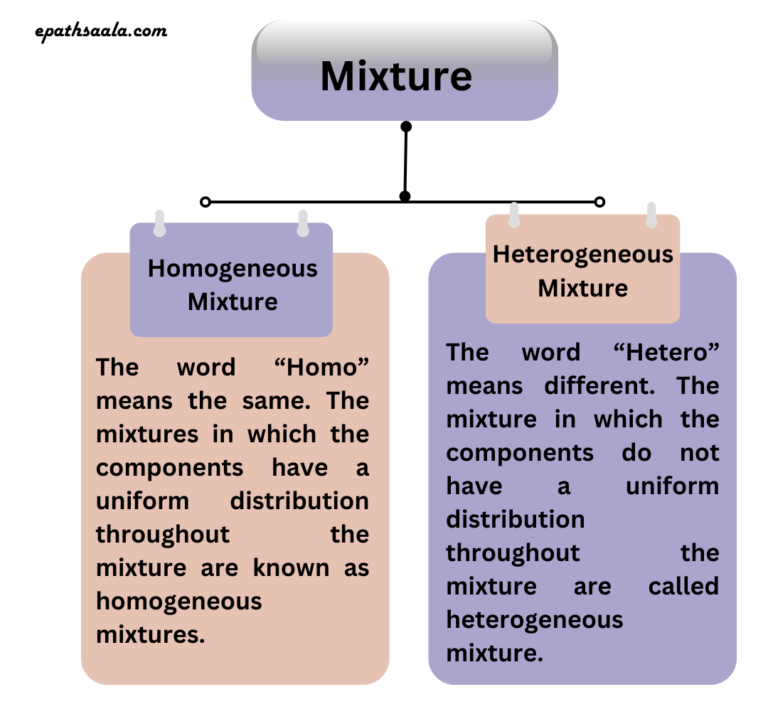
Types of mixture
- Homogeneous Mixture
- Heterogeneous Mixture
Methods of separation of substances
The substances to be separated may be particles of different sizes or materials. These may be in any three states of matter i.e., solid, liquid or gas. So, how do we separate substances mixed together if they have so many different properties?
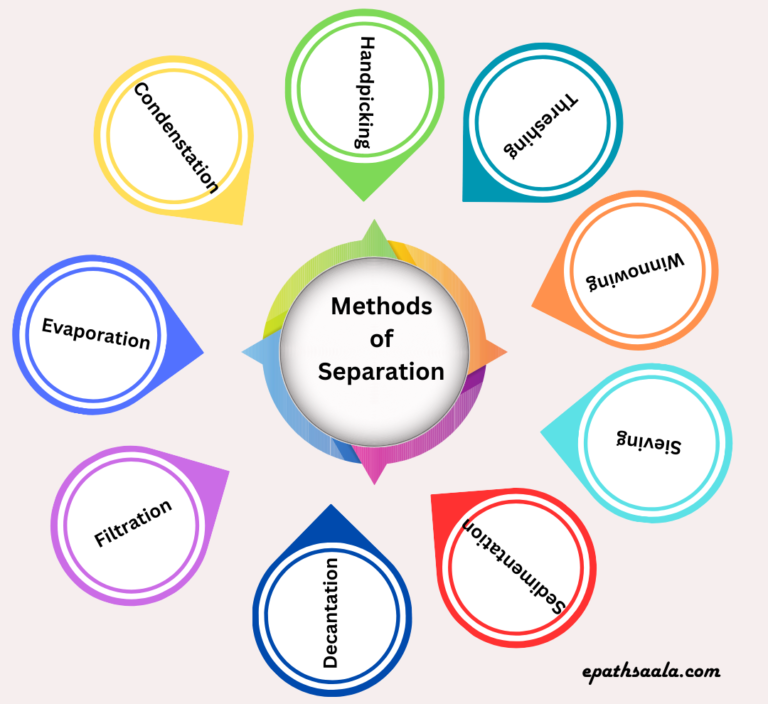
The answer is that there are some simple methods of separation of substances that are mixed together:
- Handpicking
- Threshing
- Winnowing
- Sieving
- Sedimentation
- Decantation
- Filtration
- Evaporation
- Condensation
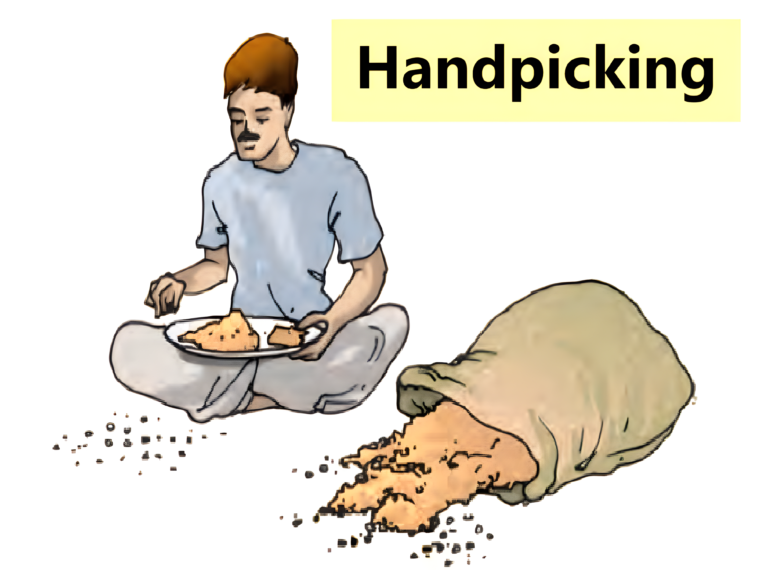
Handpicking
- In this method, a slightly larger-sized impurities like the pieces of dirt, stone and husk from wheat, rice or pulses can be separated by just picking them out with the help of a hand.
- The quantity of such impurities is usually not very large.
Threshing
- Threshing is a process that is used to separate grains from stalks etc.
- In this process, the stalks are beaten to free the grain seeds.
- Threshing can be done either manually or with the help of a machine.

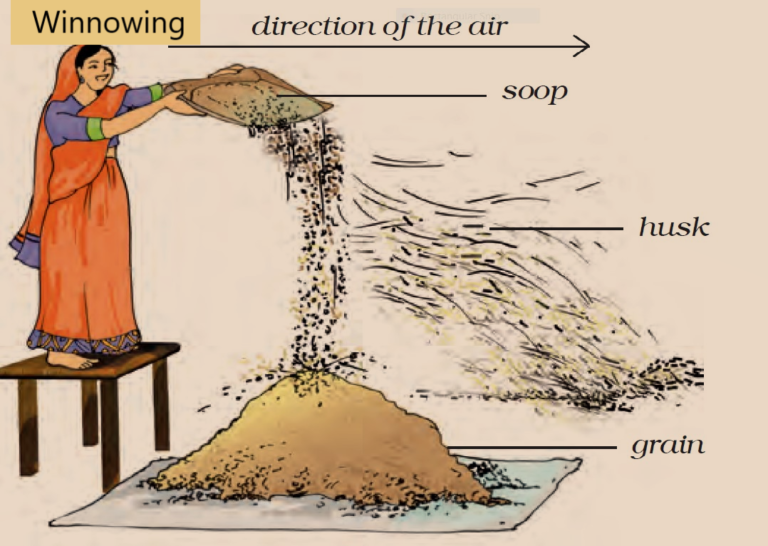
Winnowing
- Winnowing is the process of separating heavier components from the lighter components of a mixture by using wind or by blowing air.
- Farmers commonly use this method to separate lighter husk particles from heavier seeds of grain.
- In this method, the farmer stands on a raised platform and allows the mixture of grains and husk to fall down by shaking his winnowing basket continuously.
- The wind carries away the lighter husk particles whereas the heavier seeds of grain get separated and form a heap near the raised platform.
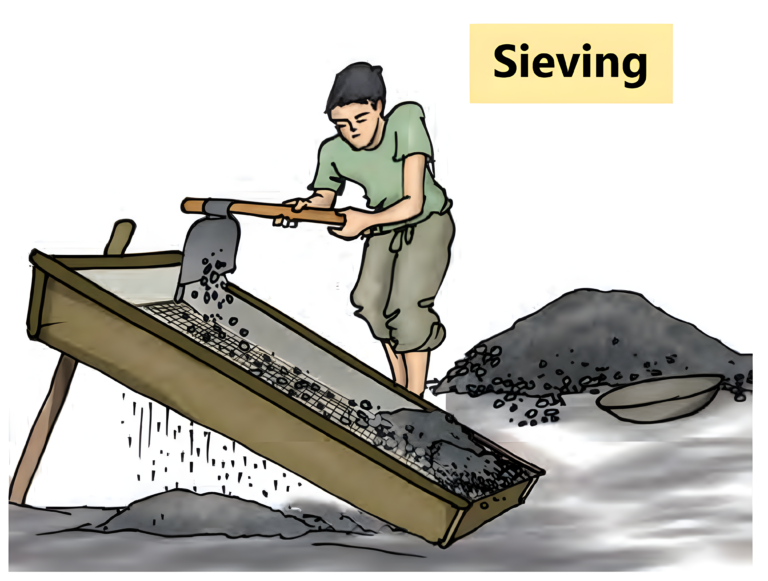
Sieving
- Sieving is a process in which fine particles are removed from the bigger particles by using a sieve.
- In this process, the holes of the sieve allow only particles of a specific size to pass through it. As a result, larger particles are retained on the sieve.
- A sieve is generally used at the construction site to separate pebbles or stones from the sand.
Sedimentation
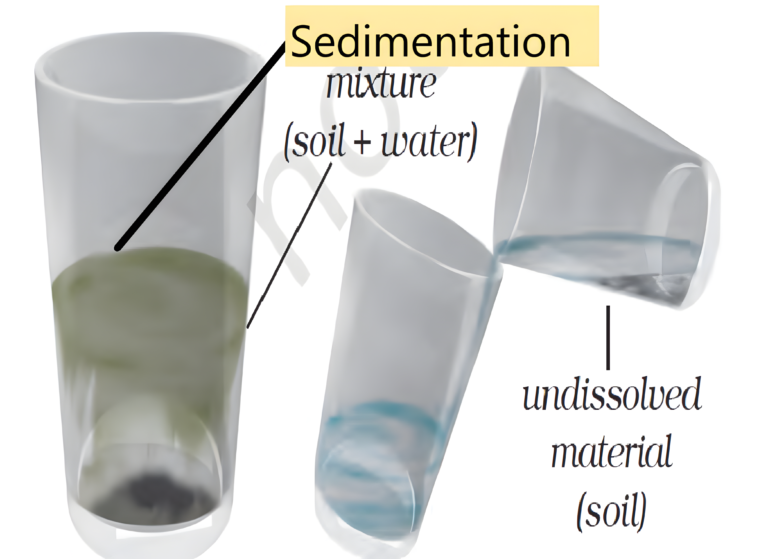
- Sedimentation is a process in which heavier particles of an insoluble solid settle down in a given liquid is called sedimentation.
- This method is used to separate dust particles or soil from water.
Decantation
- Decantation is defined as the process of separating the liquid portion of a mixture when the heavier component settles at the bottom as sediments.
- For example- In a mixture of sand and water. The sand settles down at the bottom of the jar while water is present at the upper portion of the jar. This can be separated simply by pouring the water into another container without using another separating device.

Filtration
- Filtration is the process of removing suspended particles from the liquid by passing it from the filter device.
- For example – Removing tea leaves from the tea is an example of filtration.

Evaporation
- Evaporation is the process of converting a liquid into its vapour state is called evaporation.
- Evaporation is used to get salt from the seawater.

Condensation
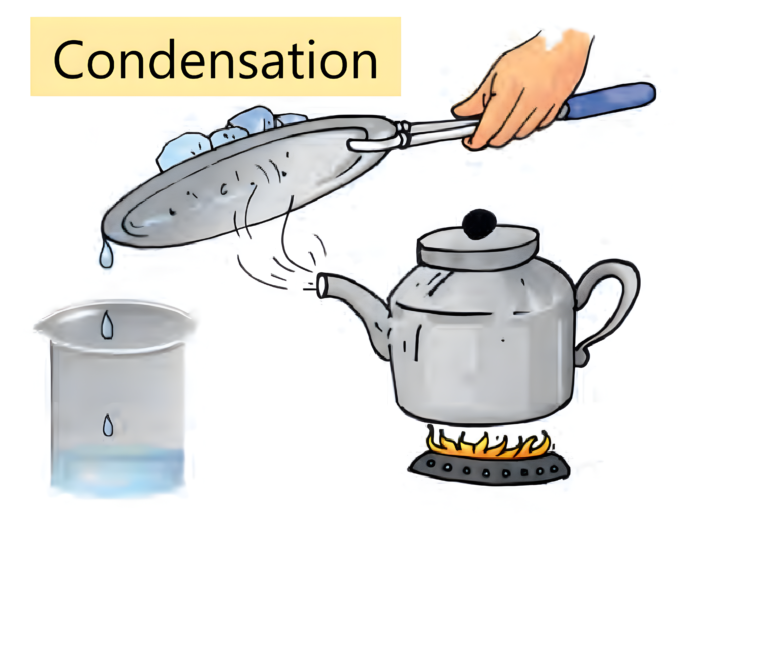
- The process of conversion of water vapour into its liquid form is called condensation.
- Condensation happens when water vapour present in the atmosphere comes in contact with a cooler surface it also cools down and turns into water droplets.
Use of more than one method of separation of substances
Earlier, we have studied some methods for separation of substances from their mixtures. Often, one method is not sufficient to separate the different substances present in a mixture. In such a situation, we need to use more than one of these methods.
Let`s take example of a mixture of sand and salt. Here we will use more than one method of separation of substances to separate sand and salt from the mixture.
- Keep the mixture of sand and salt in a beaker and add some water to it and leave it for some time.
- The sand will settle down at the bottom. This sand can be separated either by decantation or filtration.
- Now the decanted liquid contains the mixture of water and salt.
- Transfer the decanted liquid into a kettle and heat it.
- Now take a metal plate with some ice on it and placed it just above the spout of the kettle.
- When the steam comes in contact with the ice cooled metal plate the steam condenses and forms liquid water. Collect the droplets of water in a container.
- After all the water has been evaporated, only salt remains in the kettle.
- We have thus separated salt, sand and water using processes of decantation, filtration, evaporation and condensation.
Can water dissolve any amount of a substance?
No, any amount of a substance cannot be dissolved in water. The water also has certain capacity to dissolve a substance into it. This capacity differs for different substances. A substance can be completely dissolved in water upto its saturation level. Beyond that level, the water cannot dissolve a substance in it.
Let's know about solution, its components and types
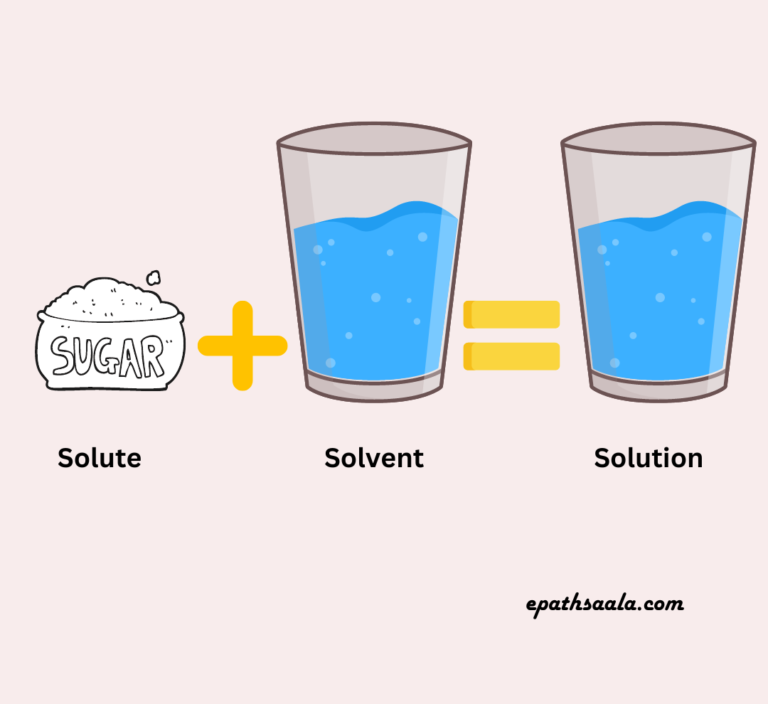
A Solution is a homogenous mixture of two or more substances. A solution has a solute and a solvent.
Solute + Solvent = Solution
- Solute:A solute is a component of a solution (usually present in lesser quantity) that dissolves in the solvent.
- Solvent: A solvent is a component of a solution (usually present in larger quantities) in which the solute dissolves.
Solution of the Exercise (Separation of substances)
1. Why do we need to separate different components of a mixture? Give two examples
Ans: We need to separate different components of a mixture:
- To remove impurities or harmful components from a mixture.
- To remove non-useful or even useful components from a mixture.
- Grain is separated from stalks during harvesting
- Tiny stones being separated from the rice or wheat.
The two examples are:
2. What is winnowing? Where is it used?
Ans: Winnowing is a method of separating heavier components from the lighter components of a mixture by using wind or by blowing air. This method of separation is used by farmers to separate lighter husk particles from heavier seeds of grain.
3. How will you separate husk or dirt particles from a given sample of pulses before cooking.
Ans: The husk or dirt particles can be separated from a given sample of pulses before cooking by using winnowing. In this method, heavier components i.e. pulses are separated from the lighter components (husk or dirt particles) by using wind or by blowing air.
4. What is sieving? Where is it used?
Ans: Sieving is a method of separation which is used to separate particles of different sizes by using a sieve. It is commonly used at the construction site to separate sand and pebbles.
5. How will you separate sand and water from their mixture?
Ans: We can separate sand and water from their mixture by the process of sedimentation and decantation.
6. Is it possible to separate sugar mixed with wheat flour? If yes, how will you do it?
Ans: Yes, it is possible to separate sugar mixed with wheat flour by sieving. The size of the sugar particles is bigger than the size of the flour particles so the flour will pass through the sieve while the sugar will be retained on the sieve due to its bigger in size.
7. How would you obtain clear water from a sample of muddy water?
Ans: Clear water can be obtained from a sample of muddy water by sedimentation, decantation and filtration method. Take the muddy water in a glass and left it undisturbed for some time. The mud in the water gets settled at the bottom of the glass. This process is called sedimentation. Now, the water is transferred to another container, called decantation. This transferred water is then filtered, called filtration, to remove the very minute particles of mud present in the water. And in this way, you will get clear water.
8. Fill up the blanks
- The method of separating seeds of paddy from its stalks is called ___________.
- When milk, cooled after boiling, is poured onto a piece of cloth the cream (malai) is left behind on it. This process of separating cream from milk is an example of ___________.
- Salt is obtained from seawater by the process of ___________.
- Impurities settled at the bottom when muddy water was kept overnight in a bucket. The clear water was then poured off from the top. The process of separation used in this example is called ___________.
- threshing
- filtration
- evaporation
- sedimentation and decantation
9. True or false?
- A mixture of milk and water can be separated by filtration.
- A mixture of powdered salt and sugar can be separated by the process of winnowing.
- Separation of sugar from tea can be done with filtration.
- Grain and husk can be separated with the process of decantation.
- False – A mixture of milk and water cannot be separated by filtration because milk and water are miscible.
- False – A mixture of powdered salt and sugar cannot be separated by the process of winnowing as the powdered salt and sugar are of the same size and weight.
- False – Separation of sugar from the tea cannot be done by filtration because sugar is soluble in tea.
- False – Grain and husk can be separated by winnowing but not by decantation process.
10. Lemonade is prepared by mixing lemon juice and sugar in water. You wish to add ice to cool it. Should you add ice to the lemonade before or after dissolving sugar? In which case would it be possible to dissolve more sugar?
Ans: As we know, solubility of a substance decreases with decrease in temperature. Therefore, we should add sugar first and then ice. If ice is added first, then the temperature of the lemonade decreases and dissolving sugar in cold water is difficult. Therefore, ice should be added to lemonade after dissolving the sugar.