Heat-Class 7-Science-NCERT solutions
NCERT notes alongwith solutions for class 7 science “Heat” are provided here. The notes as well as solutions are very much helpful for the students to understand the topics. The solutions will be handy for quickly completing the homework and preparing for exams.
Table of Contents
ToggleIntroduction: Heat
- Heat is not a matter. It doesn’t occupy space. It has no weight. Like light, sound and electricity, heat is a form of energy.
- Heat raises the temperature of a thing by causing the molecules in that thing to move faster.
- The SI unit of heat is Joule. The other unit of heat is calorie.
- In short, Heat is the total kinetic energy of constituent particles of objects.
Source of Heat
The main sources of heat are:
- Sun
- combustion (Burning)
- Friction
- Electricity
HOT AND COLD
We often determine heat by touching objects. But is our sense of touch reliable? The answer will be certainly not. Then, how do we find out how hot an object really is? The answer will be – Temperature.
A reliable measure of the hotness of an object is its temperature.
Temperature
- The measurement of warmness or coldness of a substance is known as its Temperature.
- SI unit of temperature is kelvin (K).
Units of Temperature
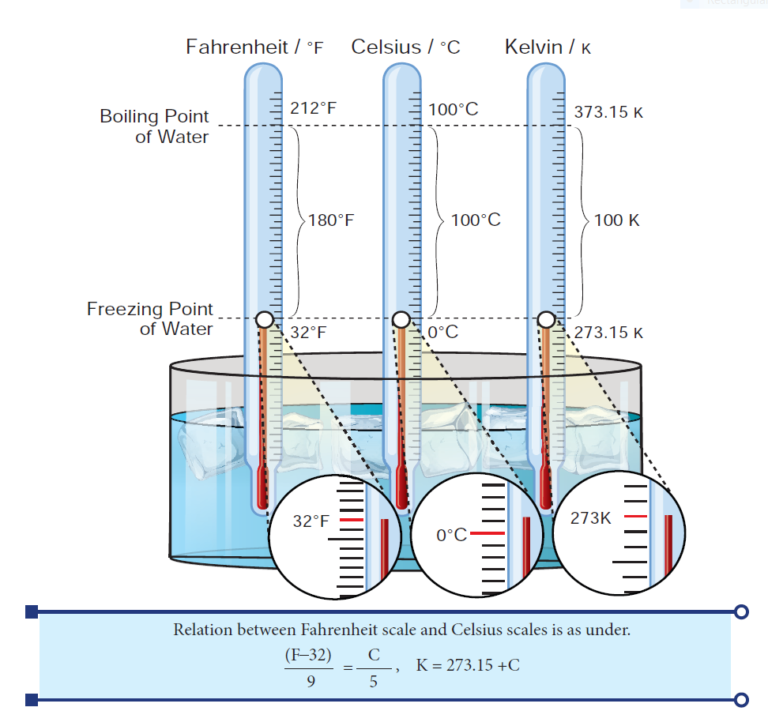
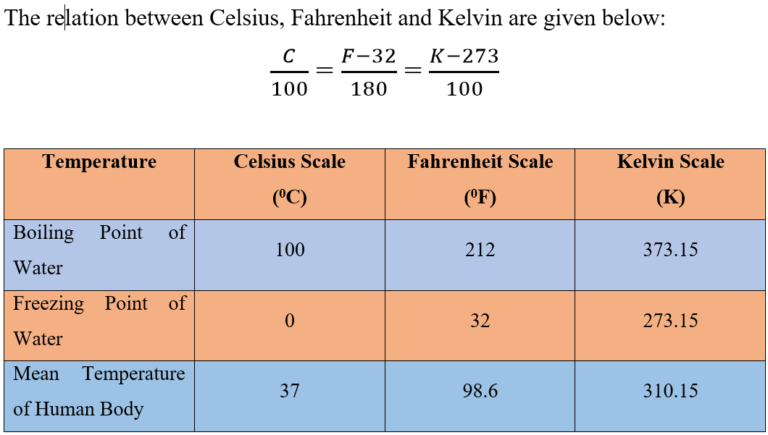
- There are three units which are used to measure the temperature: Celsius, Fahrenheit and Kelvin.
- Celsius: Celsius is written as °C and read as degree celsius. For example – 20°C; it is read as twenty degree Celsius. Celsius is called as Centigrade as well.
- Fahrenheit: Fahrenheit is written as °F for example 25°F; it is read as twenty-five degree Fahrenheit.
- Kelvin: Kelvin is written as K. For example: 100K; it is read as hundred Kelvin. The SI unit of temperature is kelvin (K).
MEASURING TEMPERATURE
- A thermometer is the most common instrument to measure temperature.
- In a thermometer, when liquid gets heat, it expands and when it is cooled down, it contracts.
- Basically, there are four types of thermometers as shown in the picture given below:
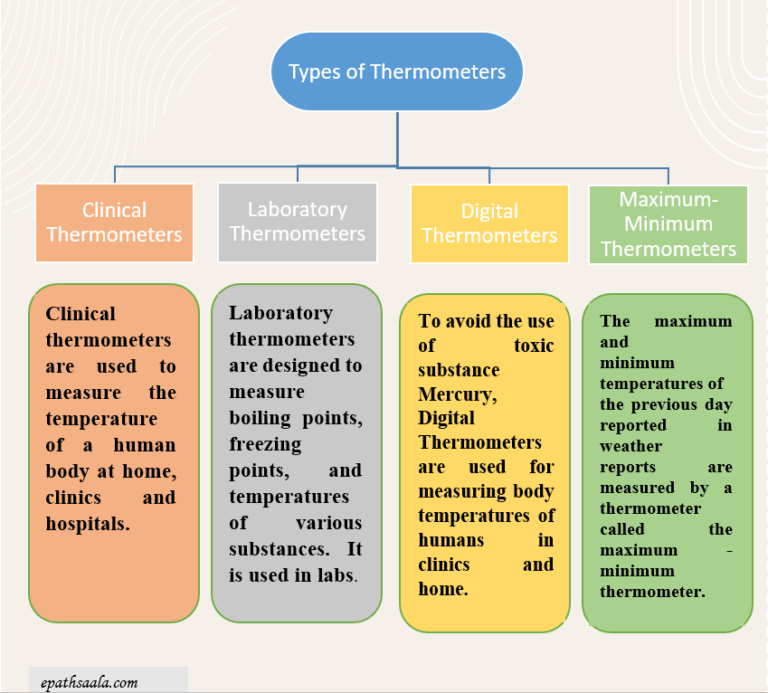
Clinical Thermometer
- The thermometer that measures our body temperature is called a clinical thermometer.
- Clinical thermometers are used to measure the temperature of a human body at home, clinics and hospitals.
- A clinical thermometer consists of a long, narrow, uniform glass tube. It has a kink as well as a bulb at one end.
- All clinical thermometers have a kink that prevents the mercury from flowing back into the bulb when the thermometer is taken out of the patient’s mouth, so that the temperature can be noted conveniently.
- The bulb of the thermometer contains mercury. Outside the bulb, a small shining thread of mercury can be seen.
- There are temperature scales on either side of the mercury thread, one in Celsius scale and the other in Fahrenheit scale. Since the Fahrenheit scale is more sensitive than the Celsius scale, body temperature is measured in °F only.
- A clinical thermometer indicates temperatures from a minimum of 35°C or 94°F to a maximum of 42°C or 108°F.
How to read a thermometer?
- First sterilize the thermometer, preferably with an antiseptic solution.
- Hold it firmly and give it a few jerks. So, the jerks will bring the level of mercury below 35°C.
- Now place the bulb of the thermometer under your tongue or arm.
- The body heat leads the mercury inside the tube to expand and, it flows through the capillary cord at a particular level.
- After one minute, take the thermometer out. The kink restricts the mercury from flowing back after the thermometer is taken out from the patient’s mouth.
- Now hold the thermometer vertically to your eyes and carefully note down the level of mercury. This is your body temperature.
- The temperature should always be stated with its unit, °C or Fahrenheit (°F).
- The normal temperature (average body temperature of a large number of healthy persons) of the human body is 37°C.
Why does the mercury not fall or rise in a clinical thermometer when taken out of the mouth?
A kink near the bulb prevents mercury levels from falling on its own.
Precautions to be observed while using a clinical thermometer
- The thermometer should be washed before and after use, preferably with an antiseptic solution.
- Ensure that before use the mercury level is below 35°C.
- Read the thermometer keeping the level of mercury along the line of sight.
- Handle the thermometer with care. If it hits against some hard object, it can break.
- Don’t hold the thermometer by the bulb while reading it.
LABORATORY THERMOMETER
- Laboratory thermometers are designed to measure boiling points, freezing points, and temperatures of various substances. It is usually used in labs for scientific research.
- The stem and the bulb of a lab thermometer are longer when compared to that of a clinical thermometer and there is no kink in the lab thermometer.
- The range of a laboratory thermometer is generally from –10°C to 110°C.
- The most common types of thermometers are:
- Liquid-in-glass thermometer
- Bimetallic strip thermometer
- Electronic thermistor thermometer
- Infrared (IR) thermometer.
Why Mercury is used in the thermometer?
Mercury is used in thermometers because it has several properties that make it ideal for this purpose. These properties are:
- Its expansion is uniform. (For equal amounts of heat it expands by equal lengths.)
- It is opaque and shining.
- It does not stick to the sides of the glass tube.
- It is a good conductor of heat.
- It has a high boiling point (357°C) and a low freezing point (−39°C). Hence, a wide range of temperatures can be measured using a mercury thermometer.
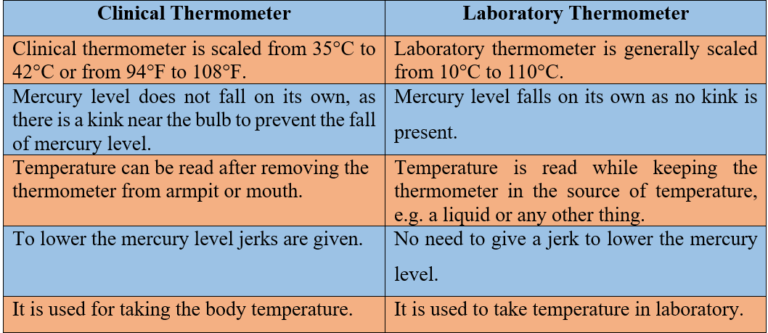
Difference between Clinical and Laboratory Thermometer
Relations between the Celsius, Fahrenheit and Kelvin
TRANSFER OF HEAT
Heat flows from a hotter object to a colder object. There are three ways of heat transfer are:
• Conduction
• Convection
• Radiation
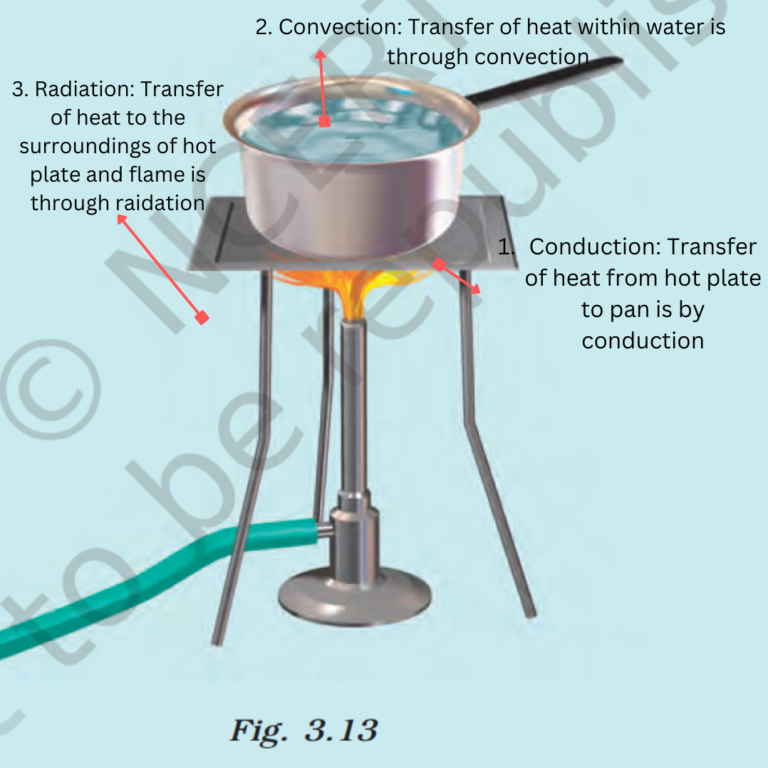
Conduction
Conduction is the process of transfer of heat in solids from the region of higher temperature to the region of lower temperature. In solids, generally, the heat is transferred by the process of conduction.
Convection
- Convection – Convection is the mode of transfer of heat by the actual movement of particles of the medium. The convection process takes place in liquids and gasses. Land breeze and Sea breeze is an example which shows that the air gets heated through convection.
- Mode of heating water through convection – When water is heated, the water near the flame gets hot. Hot water rises up. The cold water from the sides moves down towards the source of heat. This water also gets hot and rises and water from the sides moves down. This process continues till the whole water gets heated. This mode of heat transfer is known as convection.
Land and Sea Breeze
- Sea Breeze: During the day, the land gets heated faster than the water. The air over the land becomes hotter and rises up. The cooler air from the sea rushes in towards the land to take its place. The warm air from the land moves towards the sea to complete the cycle. The air from the sea is called the sea breeze.
- Land Breeze: During the night, reverse process takes place. The land gets cooled faster than the water. The hot air over the sea rises up while the cooler air of the land blow towards the sea to take its place.
Radiation
- Radiation is the mode of transfer of heat from one place to another without heating the intervening medium. The transfer of heat by radiation does not require any medium. It can take place whether a medium is present or not.
- Heat energy from the Sun reaches the Earth through this form of heat transfer i.e. radiation
Conductor and Insulators
- Conductors: The materials which allow heat to pass through them easily are conductors of heat. Mostly, metals are good conductors of heat. For example- aluminium, iron and copper.
- Insulators: The materials which do not allow heat to pass through them easily are Insulators. Insulators are poor conductors of heat such as water, air, plastic and wood.
Why we wear woollen clothes in winter?
Wool is a poor conductor of heat. Moreover, there is air trapped in between the wool fibres. This air prevents the flow of heat from our body to the cold surroundings. So, we feel warm.
Exercises
1. State similarities and differences between the laboratory thermometer and the clinical thermometer.
Sol: Similarities
- Both thermometers are made up of long narrow uniform glass tubes.
- Both thermometers have a bulb at one end.
- Both contain mercury in their bulbs.
- Both use the Celsius scale to measure the temperature.
Differences

2. Give two examples each of conductors and insulators of heat.
Sol:
- Conductors:– Aluminium & Silver
- Insulators:– Wood & plastics
3. Fill in the blanks:
- The hotness of an object is determined by its __________.
- Temperature of boiling water cannot be measured by a _____________ thermometer.
- Temperature is measured in degree ______________.
- No medium is required for transfer of heat by the process of __________.
- A cold steel spoon is dipped in a cup of hot milk. Heat is transferred to its other end by the process of ______________.
- Clothes of ______________ colours absorb more heat better than clothes of light colours.
Sol:
- Temperature
- Clinical
- Celsius
- Radiation
- Conduction
- Dark
4. Match the followings
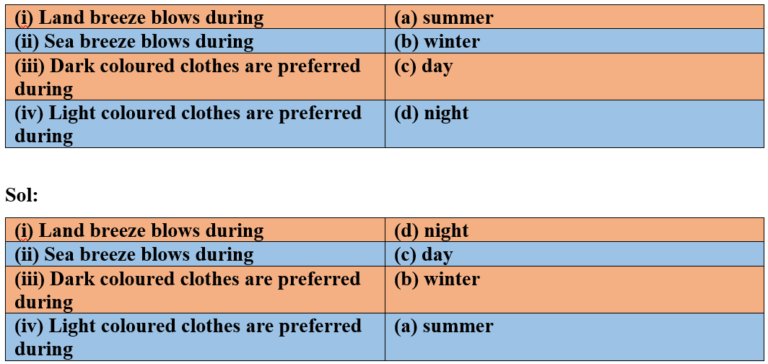
5. Discuss why wearing more layers of clothing during winter keeps us warmer than wearing just one thick piece of clothing.
Sol:
We prefer wearing more layers of clothing than just one thick of clothing in winter because the air gets trapped in between the various layers of clothing. Being a poor conductor of heat, the air prevents the flow of heat from our body to the cold surroundings. Thus, it prevents the loss of heat from our body. So, we feel warm.
6. Look at Fig. 3.13. Mark where the heat is being transferred by conduction, by convection and by radiation.
Sol:
7. In places of hot climate it is advised that the outer walls of houses be painted white. Explain.
Sol:
In places of hot climate, it is advised that the outer walls of houses be painted white because white-coloured objects absorb less heat produced by sunlight and reflect more in comparison with other coloured objects. As a result, the temperature inside the house will not increase very much.
8. One litre of water at 30°C is mixed with one litre of water at 50°C. The temperature of the mixture will be (a) 80°C (b) more than 50°C but less than 80°C (c) 20°C (d) between 30°C and 50°C
Sol:
(d) between 30°C and 50°C. This is because there is a transfer of heat from an object with a high temperature to an object with a low temperature. So, heat will flow from one litre of water at 50°C to one litre of water at 30°C. Hence, the temperature of the mixture will come in between 30°C and 50°C.
9. An iron ball at 40°C is dropped in a mug containing water at 40°C. The heat will (a) flow from the iron ball to water. (b) not flow from iron ball to water or from water to iron ball. (c) flow from water to iron ball. (d) increase the temperature of both.
Sol:
(b) not flow from iron ball to water or from water to iron ball. The reason is heat always flow from an object with a higher temperature to an object with a lower temperature. In this case, the temperature of iron ball and water is 40°C. So, no heat will flow in between these two objects.
10. A wooden spoon is dipped in a cup of ice cream. Its other end (a) becomes cold by the process of conduction. (b) becomes cold by the process of convection. (c) becomes cold by the process of radiation. (d) does not become cold.
Sol:
(d) does not become cold. The reason is wood is a bad conductor of heat which means it does not allow heat to pass through it. As a result, when the wooden spoon is dipped in a cup of ice-cream. Its other end will not become cold.
11. Stainless steel pans are usually provided with copper bottoms. The reason for this could be that (a) copper bottom makes the pan more durable. (b) such pans appear colourful. (c) copper is a better conductor of heat than the stainless steel. (d) copper is easier to clean than the stainless steel.
Sol:
(c) copper is a better conductor of heat than the stainless steel. Reason: As we know copper is a better conductor of heat than stainless steel which means that the heat transfers from the burner to the food inside the pan having a copper bottom would be easier than using a pan with a stainless steel bottom. This will save fuel which will be used in cooking food.